CancerDetect® Test for Oral & Throat Cancer
CancerDetect® Oral & Throat is the first at-home test of its kind to detect biomarkers associated with early-stage oral and throat cancer. This simple-to-use yet revolutionary test takes only a few minutes to collect and mail a sample and includes healthcare provider guidance when needed. Eligible individuals can now test with the confidence of high accuracy to detect biomarkers associated with oral and/or throat cancer.
-
 Detect biomarkers associated with early-stage oral & throat cancers
Detect biomarkers associated with early-stage oral & throat cancers -
 Substantially higher accuracy over current screening methods
Substantially higher accuracy over current screening methods -
 Easy-to-use at-home test and guided experience
Easy-to-use at-home test and guided experience -
 Healthcare provider consultation is available when needed
Healthcare provider consultation is available when needed
* In a recent meta-analysis, across 14 studies, the summary estimate for sensitivity of conventional oral examination was found to be 71% (95% CI: 57%–81%), and the specificity was 85% (95% CI: 68%–94%).
Order testTest Details
Saliva collection at home
This test detects molecular features associated with Oral Cancer (Oral Squamous Cell Carcinoma or OSCC), and/or Throat Cancer (Oropharyngeal Squamous Cell Carcinoma or OPSCC) in saliva samples.
-
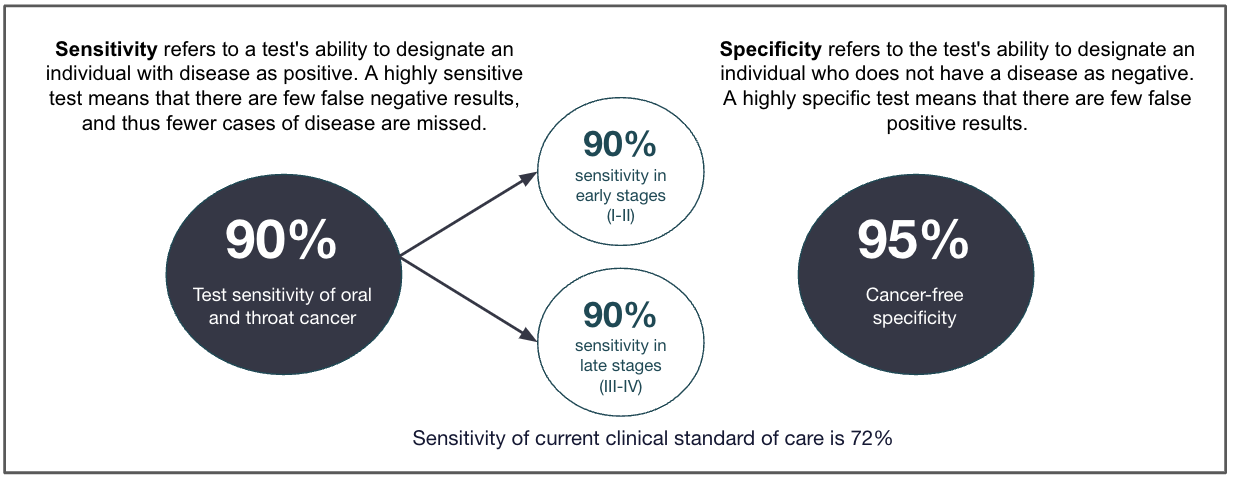
Cancer-free specificity is ≥ 95% *
-
OSCC sensitivity is ≥ 90% *
-
OPSCC sensitivity is ≥ 90% *
Sensitivity refers to a test's ability to designate an individual with a disease as positive. A highly sensitive test means that there are few false-negative results, and thus fewer cases of the disease are missed. Specificity refers to a test's ability to designate an individual who does not have a disease as negative. A highly specific test means that there are few false-positive results, and thus few false alarms.1
- AS FEATURED IN
-
-
-

Oral and Throat Cancer Detection Test built on
Viome platform, designated Breakthrough Device for accelerated Review by FDA
What’s included with CancerDetect® - Oral & Throat

Instructions to guide your testing experience.

Everything you need to collect your saliva sample at home

A digital report that you can print and review your results
Prepaid First Class USPS label and mailing envelope

Consultation with a healthcare provider to help you better understand
At-risk individuals
Major risk factors for the development of oral and throat cancer include tobacco use and alcohol consumption.
-
75% of oral cancers in the United States are attributable to tobacco use and alcohol consumption.
-
Tobacco use can include consuming tobacco products by smoking, chewing, vaping, etc.
-
Older age, HPV (human papillomavirus) infection, and excess body weight are additional risk factors for oral and throat cancer, and the risk increases more rapidly after 50 years of age.
-
Even though throat cancers (OPSCC) are the most common HPV-related cancers in the United States, no early detection strategy for OPSCC is in place for HPV-positive individuals.14
A solution for earlier detection
Oral cancer is a major subtype of head and neck cancers.
-
40,000 new cases of oral cancers every year in the US
-
This is expected to increase by nearly two-thirds by 203510
Currently, oral cancer is hard to detect in the early stages because of the lack of effective early diagnostic tools, resulting in late diagnosis, leading to poor prognosis and low survival rates.
The performance characteristics of visual screening are operator-dependent, with sensitivity ranging from 25% to 100%, with the study most generalizable to the United States demonstrating a sensitivity of 74%.11
NEWS ARTICLE
Oral and Throat Cancer Detection Test built on Viome platform, designated Breakthrough Device for accelerated Review by FDA
Our testing delves beyond your DNA to explore the dynamic world of gene expression (RNA), offering a real-time snapshot of your health's inner workings. With our unique technology, we unlock personalized insights tailored specifically to your current needs, pinpointing the exact foods, supplements, and biotics that benefit you most. Let us steer you towards making informed health decisions based on true data, moving away from one-size-fits-all guidance and guesswork.

Early Testing Process
CLIA certified
Your sample analysis is performed in a US laboratory that is certified to meet CLIA standards. A CLIA-certified lab must meet certain quality standards and ensure the accuracy and reliability of your results.
Privacy & data
To ensure the confidentiality of your data, we separate users’ personally identifiable information (PII) from protected health information (PHI) and use multiple layers of encryption and access protection. We do not provide your information or results to employers or health insurance. Viome will never sell your data.


Inside your test collection kit
The underestimated challenge of oral and throat cancer detection
While medical advances are making it easier to live longer and better lives, we still fail to detect oral cancer early. Shocking statistics reveal a staggering 62% surge in global oral cancer cases by 2035 due to different factors, including a lack of new diagnostic and detection tools in clinical practice in the last 40 years.
In current clinical practice, diagnosing oral cancer relies on the expertise of various healthcare providers (dentists, dental hygienists, primary physicians). Screening for oral and throat cancer involves a visual and tactile check. However, assessing the oral cavity and throat this way is akin to exploring the ocean's depths with only a flashlight and hands. While you might glimpse prominent features and feel surfaces, a world of complexity and hidden details escapes these limited senses. To gain complete insight, embracing easy-to-use advanced tools and techniques is essential.
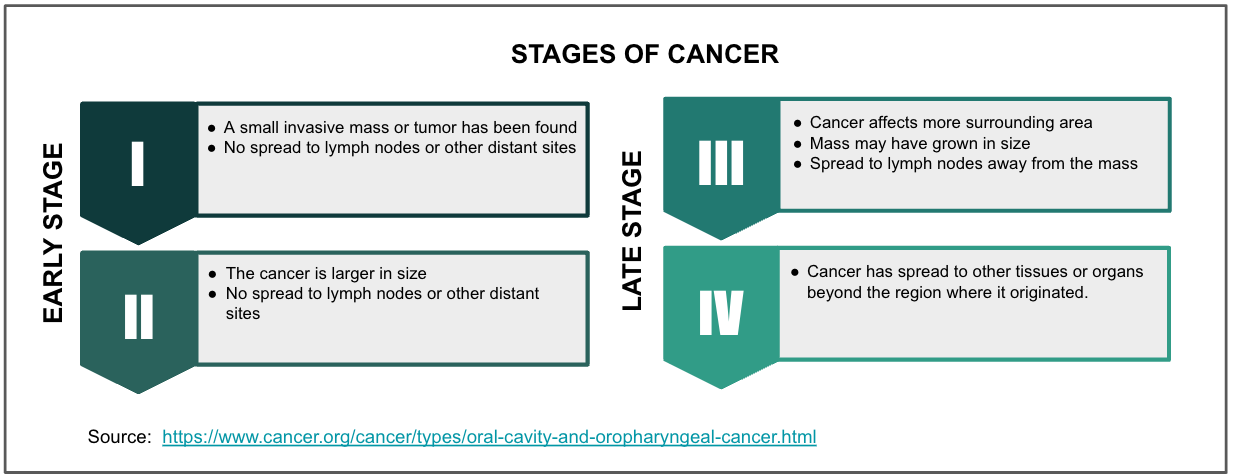
In addition, the visual and tactile examination is subjective and prone to examiner bias. In fact, this technique is not very sensitive* - the number of wrongly diagnosed positive cases (false negatives) is still high - making it harder to identify precancerous oral lesions. The absence of symptoms in the early stages (I-II) of the disease can also delay the diagnosis. More than 70% of oral cancer diagnoses are not made until the disease is in stage III or IV. At these later stages, the five-year survival rate drops to less than 50%. Unfortunately, only 28% of patients with oral cancer in the U.S. are diagnosed at an early stage, when the malignancy is localized, treatable, and the 5-year survival is high.
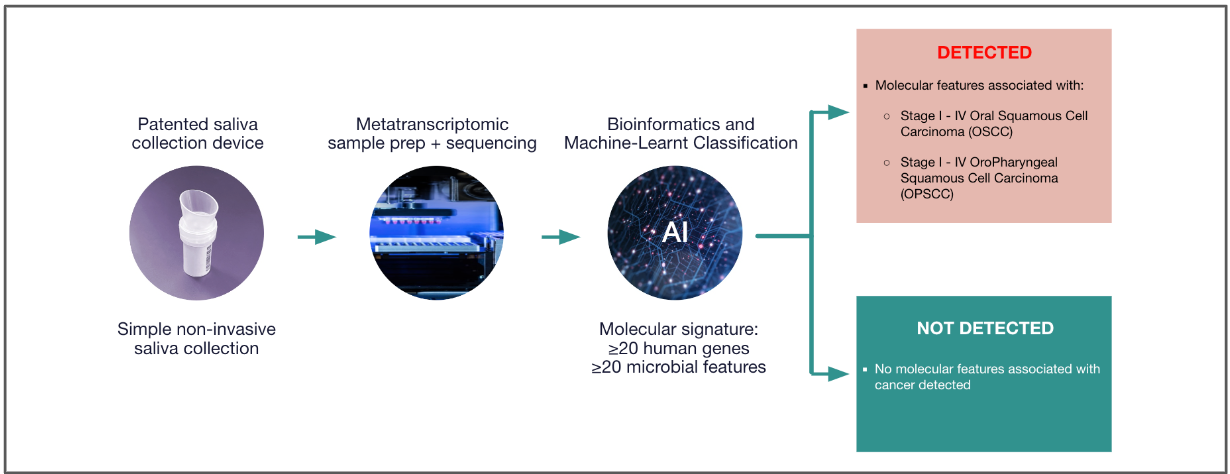
This single-use kit provides materials and instructions for collecting and preserving genetic material (RNA) from a saliva sample which will be analyzed to detect molecular features associated with Oral Cancer and/or Throat Cancer. Like a powerful telescope charting the depths of the galaxy, the test embarks on a journey of RNA sequencing analysis, uncovering hundreds of human and microbial RNA features. These features serve as essential markers, guiding the way toward the early detection of oral and throat cancer.
It is an easy-to-use saliva-based detection test, with 95% specificity and sensitivity of 90% for both oral squamous cell carcinoma (the most common type of oral cavity cancer) and oropharyngeal squamous cell carcinoma (the most common type of throat cancer). This test has the potential to save many lives, and was designated Breakthrough Device for accelerated Review by FDA.
FAQs
According to Viome, CancerDetect Test offers ~90% sensitivity for both oral (OSCC) and throat (OPSCC) cancers and ~95% specificity. In contrast, traditional visual exams show ~71% sensitivity and ~85% specificity, making CancerDetect substantially more reliable for early-stage detection.
The test is a laboratory-developed test (LDT) performed in a CLIA-certified lab. It has received FDA Breakthrough Device designation indicating its potential to address unmet needs but it is neither cleared nor approved by the FDA. It is intended as a screening aid, not a definitive diagnostic tool.
A 2023 peer-reviewed study found ~90% sensitivity and ~94% specificity for OSCC/OPSCC detection via salivary RNA markers validated in both early and late-stage cancers. These findings reinforce Viome’s claims and demonstrate real-world performance.
Given the rise of oral cancer, Dentulu recommends this test for all adults, especially those 18+ with risk factors such as tobacco use (current or past), heavy alcohol use, HPV exposure, or suspicious oral symptoms.
Predictable and user-friendly collect saliva at home using the provided kit; no dietary, medication, or lifestyle modifications required. Ship via prepaid label; analysis occurs in a CLIA lab. Results are delivered through a secure portal, with a provider consultation if markers are detected.
Detected: Molecular signatures associated with oral/throat cancers were identified. You will receive a phone consultation from a licensed provider to discuss confirmatory steps.
Not Detected: No immediately associated biomarkers found but this does not rule out cancer. Continue regular screenings and discuss retesting intervals with your provider.
See a specialist (e.g., ENT, oncologist) promptly for confirmatory evaluation such as imaging or biopsy. The test is not diagnostic but a guide for early detection and timely referral and intervention.
Yes, your full report is available in PDF form via your secure portal. If you cannot be reached after a positive result, a certified mail is sent to ensure continuity of care.
Retesting depends on risk factors. Many clinicians recommend annual reassessment, particularly for individuals with persistent risk behaviors or symptoms. There are no downsides to testing annually.
Dentulu is more than a distributor for Viome products, we are a nationwide network of licensed dental providers that help oversee preventive testing and treatments at home before problems become worse. Ordering through Dentulu ensures that your test results are reviewed by a qualified dental professional, who can guide you on next steps and coordinate referrals to local dentists, ENTs, or oncologists if necessary. This added layer of professional oversight is unique to Dentulu and helps patients feel confident and supported throughout the process.
Dentulu operates the largest HIPAA-compliant Teledentistry network in the U.S., allowing patients in both urban and rural communities to access advanced oral cancer screening without leaving their homes. Our digital platform bridges the gap between patients, dental providers, and medical specialists removing barriers like travel distance, scheduling conflicts, and cost.
Dentulu is built by dentists for patients and providers. Dentulu was awarded the Best of Class Technology as the best Teledentistry company at the American Dental Association two years in a row. Unlike general telehealth platforms, Dentulu focuses on dentistry, oral health, and the connection between oral and systemic disease. We pioneered consumer technologies like the MouthCam® intraoral camera and offer a complete marketplace of vetted dental products and services overseen by some of the nations leading dental professions. Partnering with Viome on CancerDetect extends our mission to make preventive, life-saving solutions widely available.
Yes. Dentulu partners with thousands of licensed dentists nationwide. If your test shows a “Detected” result, Dentulu will connect you to a nearby dental or medical professional for timely follow-up care. This ensures continuity of care and eliminates the risk of patients being left on their own after receiving results.
Through Dentulu’s professional portal, dental providers and physicians can seamlessly order CancerDetect kits for their patients, access training and clinical resources, and view results securely. Dentulu also helps practices market this service, making it easy for dentists to expand preventive care offerings and strengthen their role in overall health.
Both. Patients can order tests directly through Dentulu, while dental offices and DSOs can partner with us to bring CancerDetect and other innovative products into their practices. Dentulu’s B2B ecosystem connects providers with leading technologies, offering streamlined logistics, compliance support, and revenue-generating opportunities.
Dentulu’s platform is fully HIPAA-compliant and designed to protect patient health information. Results are delivered through secure portals, accessible only by you and the licensed professionals you authorize. Dentulu never shares your information with employers, insurers, or third parties without your consent.